Polycyanurates
(Cyanate or Triazine Polymers)
Properties
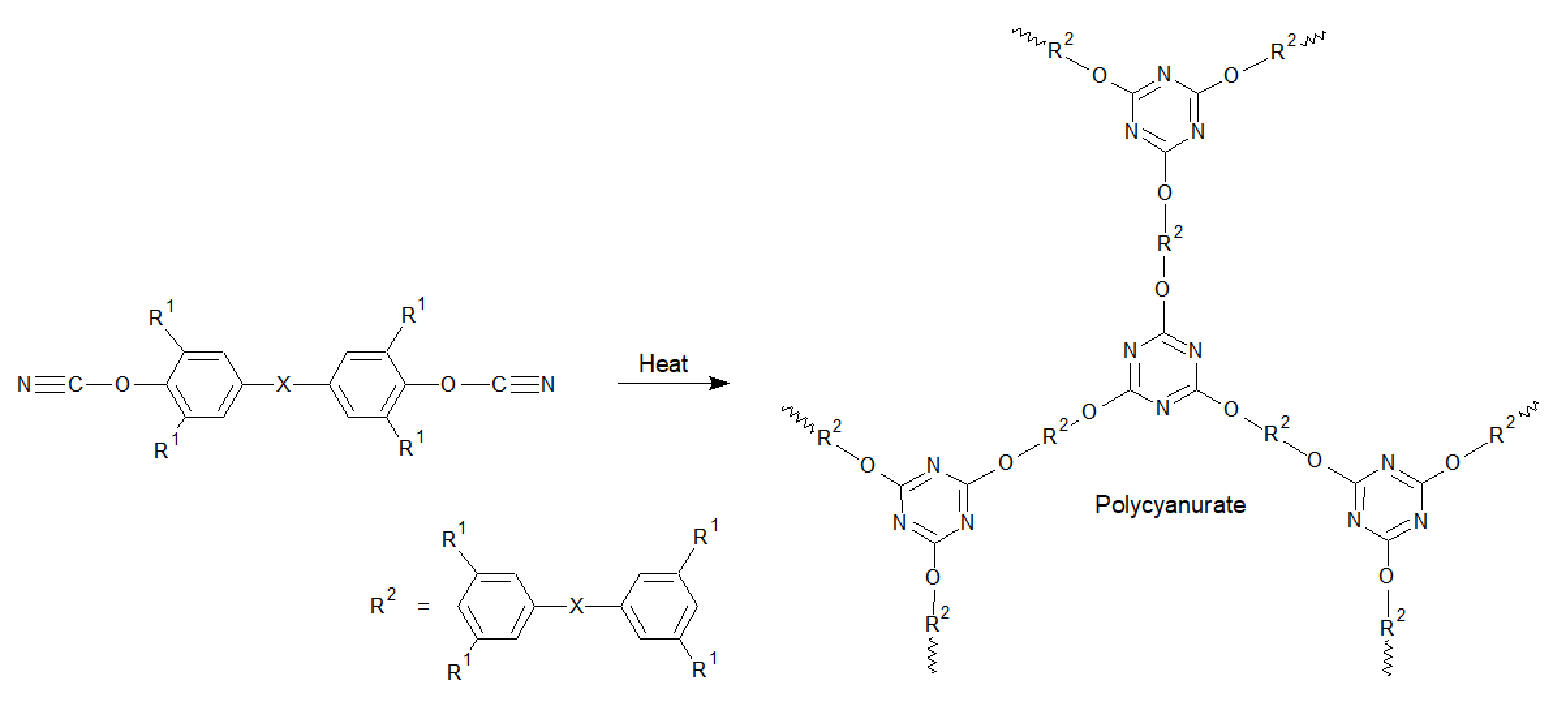
Polycyanurates are a class of high performance engineering plastics with very high thermal, oxidative, and hydrolytic stabilitiy. The uncured monomers and prepolymers, the cyanate esters and triazine resins, contain reactive cyanate functional groups that undergo an exothermic cyclotrimerization reaction in the presence of a suitable catalyst and/or when heated to form highly crosslinked network polymers the so called polycyanurates.
Polycyanurates have a very high decomposition temperature, and thus outstanding long-term thermal stability. They also possess very high glass transition temperatures. Values between 480°F and 750°F (250 - 400°C) have been reported.1,2 The high thermal stability and tensile strength of fully cured cyanate resins is provided by the 3D network structure of triazine rings and bisphenols linked by ether groups. This structure also imparts outstanding flame retardancy and chemical resistance, but make the polymer very rigid. However, when blended with functionalized thermoplastic resins, such as reactively-terminated poly(ether sulfone) and a polyester copolymers, the toughness of polycyanurates can be significantly increased.3 In fact, well formulated resin systems combine the toughness and adhesion properties of epoxies with the excellent thermal properties of toughened BMIs. Their mechanical properties are typically between those of epoxies and bismaleimides.
Polycyanurates have many other interesting properties. For example, they exhibit low moisture absorption when compared to other high temperature resins such as BMI and polyepoxides and they have very low dielectric values at high frequencies.
COMMERCIAL Cyanate Esters
Commercial grades of bisphenol-type dicyanate ester resins are available from Huntsman and Lonza. The Bisphenol E based grades have very low viscosities whereas the Bisphenol-M based resins have very low cure temperatures and the lowest dielectric constants. In some cases two or more resins are blended to lower the viscosity so that the resin can impregnate fiber filaments without heating (prepregs). The Novolac type cyanate resins have the highest thermal stability of all commercial grades and also provide excellent inherent flame retardancy.
| Cyanate Ester | Structure of Repeat Unit | Trade Name |
| Bisphenol A Dicyanate |
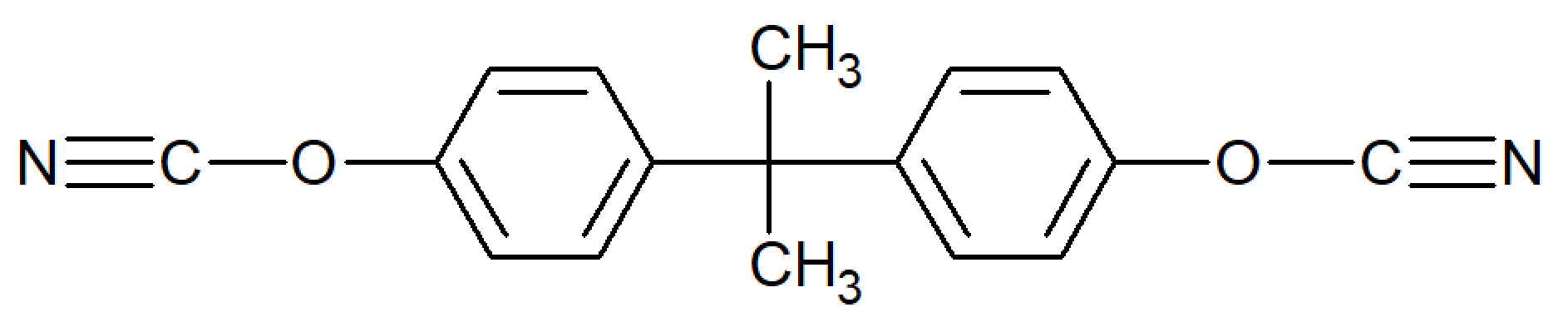
| Primaset™ BADCy (Lonza) |
| Bisphenol E Dicyanate |
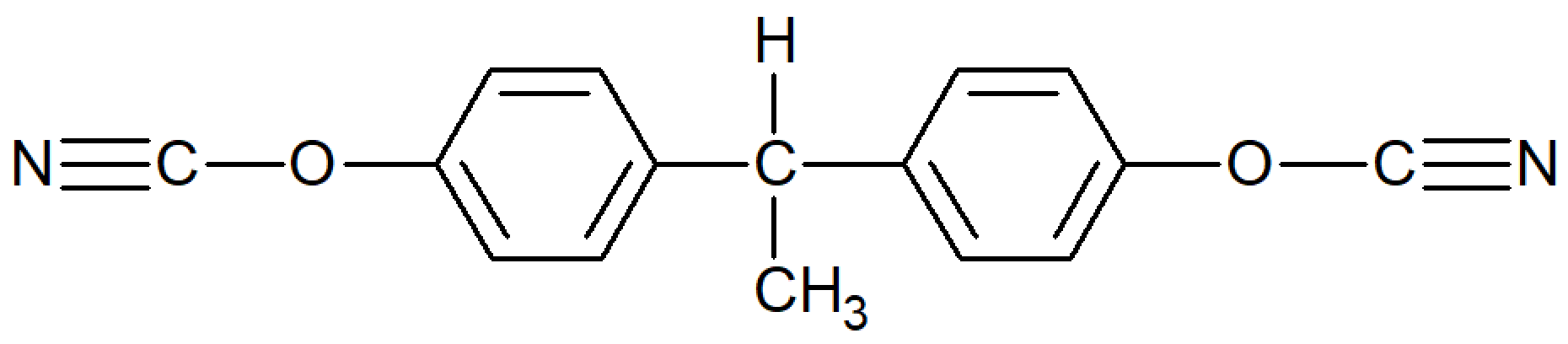
| Primaset™ LECy (Lonza) AroCy L-10 (Huntsman) |
| Tetramethyl Bisphenol F Dicyanate |
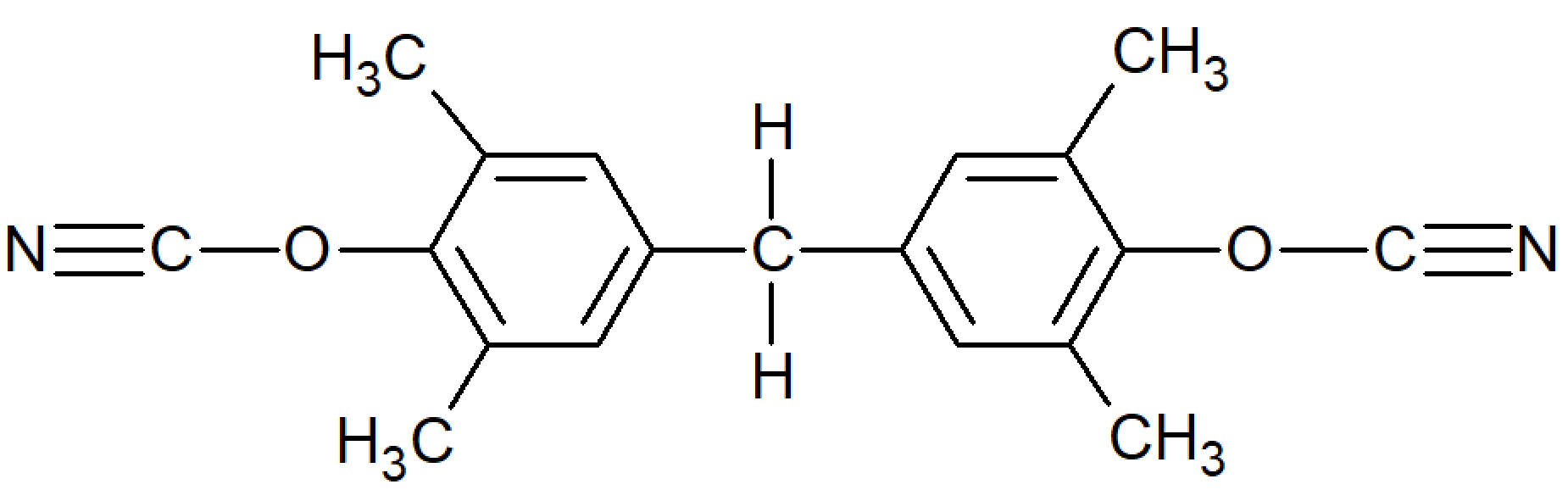
| Primaset™ METHYLCy (Lonza) |
Applications
Cyanate esters are used as binders in high-performance structural composites, mainly in the aerospace industry, because of their outstanding mechanical properties and high operating temperature. Important products include filament windings, fiber-reinforced composites, pultrusion and syntactic foams. Cyanate ester resins also find some uses in the electronic industry including electronic chip adhesives, encapsulants, and thermal interface materials. They are prefered over epoxy resins in demanding applications because they have greater thermal stability, superior dielectric properties, and much lower moisture absorption but are less frequently used due to their much higher price.
References and Notes
1G. La Delfa, W. Voigt, M. Abgottspon, Cyanate Ester Resins for Aerospace Applications, Conference Paper (2010) and Huntsman brochure
2Very high glass transition temperatures well above 300°C have been reported for Novolac-based cyanate esters.
3A.J. Kinloch, A.C. Taylor, J. of Mat. Sci., vol. 38, 65-79 (2003)